Products

GEM Premier ChemSTAT®
Introducing the new GEM Premier ChemSTAT, a rapid Basic Metabolic Panel (BMP) whole-blood analyzer with a menu customized for the Emergency Department, including lab-quality Creatinine results. Advanced connectivity with Intelligent Quality Management (iQM) ensures simplicity at the point of care (POC) and expedited time to triage and treatment. GEMweb Plus 500 Custom Connectivity simplifies and centralizes management of POC testing.
Basic Metabolic Panel. STAT.

Lab-quality, actionable results at the point of care
The GEM Premier ChemSTAT whole-blood testing system measures BMP, including lab-quality creatine, plus Hct, Lac, pH and pCO2, with calculated renal function parameters, eGFR (MDRD) and eGFR (CKD-EPI).
- Rapid results from just one sampleVenous or arterial lithium-heparinized, whole-blood samples. Results in 70 seconds, enabling rapid clinical decision-making.
- Intelligent Quality Management (iQM)Automated, real-time and continuous quality management, ensuring lab-quality results and ease of use at the POC.
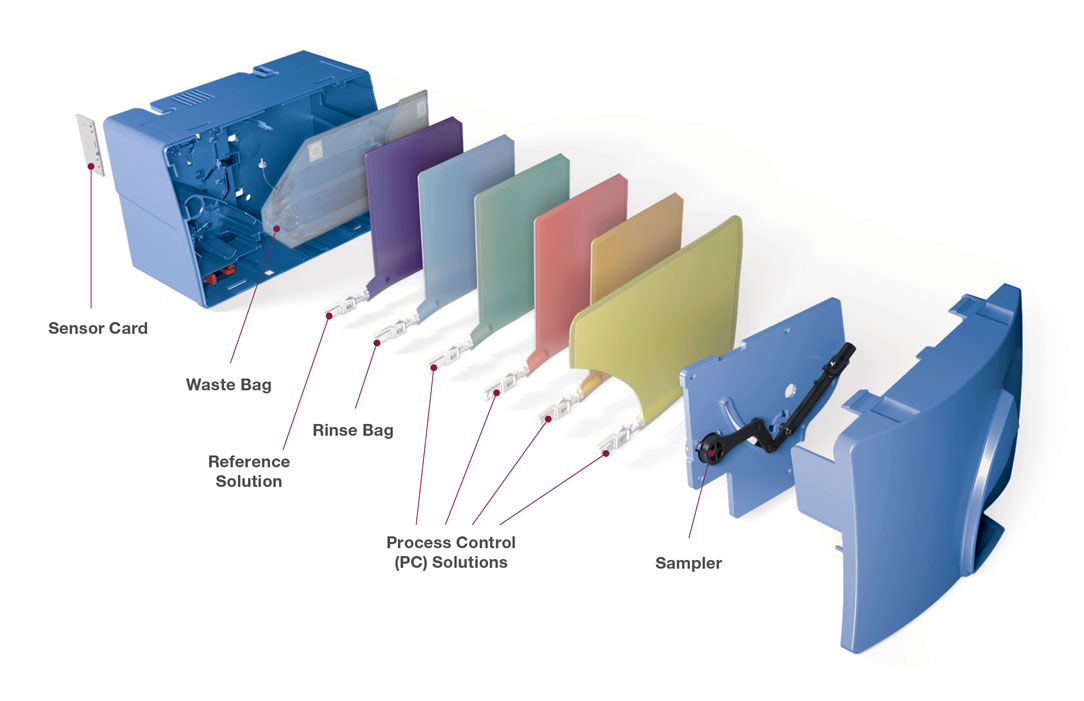
- All-in-one, multi-use cartridge (GEM PAK)Self-contained and non-refrigerated, simplifying operations at the POC.
iQM ensures quality and GEM PAK offers ultimate simplicity

Menu and PAK configurations
FLEXIBLE GEM PAK CUSTOMIZATION
Gemeten analyten
Na +, K +, Ca ++, Cl-, Glu, Crea, BUN, tCO2, Hct, Lac, pH, pCO2Afgeleide parameters (berekend)
AG, HCO3- (m), BUN / Crea ratio, BEecf, BE (B), tHb (c), Ca ++ (7.4), Osm, eGFR (MDRD) *, eGFR (CKD-EPI) **, eGFR (MDRD) *, eGFR (CKD-EPI) **Patienten monstervolume
150 μL voor het verkrijgen van Na +, K +, Ca ++, Cl-, Glu, Lac, Hct, Crea, BUN, tCO2, pH, pCO2
Beschikbare opties:
BMP: Na +, K +, Ca ++, Cl-, Glu, Crea, BUN, tCO2, Hct.
BMP Plus: Na +, K +, Ca ++, Cl-, Glu, Crea, BUN, tCO2, Hct, Lac, pH, pCO2
* De analysator levert twee eGFR-resultaten als het Crea-resultaat, de leeftijd, het geslacht en de etniciteit beschikbaar zijn: eGFRAA (MDRD) voor Afro-Amerikanen (AA) en eGFR (MDRD voor niet-AA).
** Als het Crea-resultaat, de leeftijd, het geslacht en de etniciteit beschikbaar zijn, levert de analysator twee eGFR-resultaten: eGFRAA (CKD-EPI) voor Afro-Amerikanen (AA) en eGFR (CKD-EPI) voor niet-AA.
Crea (Creatinine), AG (Anion Gap), HCO3 (Bicarbonaat), BUN / Crea (BUN / Creatinine ratio) BEecf (Baseline overmaat extracellulaire vloeistof [in vivo]), BE (B) (Baseline overmaat bloed [in vitro ]), tHb (c) (berekend totaal hemoglobine), Ca ++ (7,4) (Ca ++ genormaliseerd naar pH 7,4), Osm (osmolaliteit), eGFR (geschatte glomerulaire filtratiesnelheid), MDRD (dieetmodificatie bij nierziekte), CKD- EPI (chronische nierziekte - epidemiologische samenwerking).
GEM Premier ChemSTAT is niet in alle landen beschikbaar.
Complementary Products
GEM Premier ChemSTAT - BMP Plus - 75 test PAK
GEM Premier ChemSTAT - BMP Plus - 150 test PAK
GEM Premier ChemSTAT - BMP Plus - 300 test PAK
GEM Premier ChemSTAT - BMP Plus - 450 test PAK
ChemSTAT System Evaluator 1, 10 ampoules x 1.8 mL
ChemSTAT System Evaluator 2, 10 ampoules x 1.8 mL
ChemSTAT System Evaluator 3, 10 ampoules x 1.8 mL
ChemSTAT Hematocrit Evaluator 1, 10 ampoules x 1.8 mL
ChemSTAT Hematocrit Evaluator 2, 10 ampoules x 1.8 mL
ChemSTAT Hematocrit Evaluator 3, 10 ampoules x 1.8 mL
GEM ChemSTAT PVP 1-5, 5 levels x 5 ampoules x 1.8 mL
GEM ChemSTAT critPVP 1-4, 4 levels x 4 ampoules x 1.8 mL
Contact us directly filling out the form below.
Belgium-Luxembourg
infobnl@werfen.com
Tel: +32 (0) 800 713 37
The Netherlands
infobnl@werfen.com
Tel: +31 (0) 800 882 02 88




iQM provides rapid, quality-assured results with real-time automatic error detection, correction and documentation of all corrective actions.